Table of Contents
Chemical engineering involves complex calculations and data-driven decision-making. To speed up workflows, specialized software is often required. Python is a versatile tool for chemical engineers that has transformed the way problems are solved.
Python is open-source and comes with a wide range of powerful libraries that help solve problems efficiently and streamline workflows. In this article, we’ll explore how these Python libraries can be used to simulate chemical reactions, analyze complex process data, design new materials, and much more.
Related: Why Chemical Engineers Are Switching to These Open-Source Tools in 2025
Why Python for Chemical Engineers
Their is a significant shift towards Python in chemical engineering isn’t just a trend; it’s a strategic advantage. Here’s why:
- Automation: Python can automate repetitive calculations and data tasks.
- Data Analysis & Visualization: Large amounts of data, finding patterns, and insightful visualizations.
- Modeling & Simulation: Python is also used for creating custom simulations for reactions, heat flow, and more.
- Cost-Effective: Python and its libraries are free to use.
- Interoperability: Python can also be connected with software you already use.
- Community & Resources: Python has a huge documentation and community
Note: Python can be used on various platforms, including personal computers with Anaconda, Jupyter Notebook, or VS Code. A cloud-based option, Google Colab offers a free, browser-based environment with built-in libraries and GPU support which make it ideal for running code without installation and easy collaboration.
Here are the 8 Python Libraries Every Chemical Engineer Should Know for Faster Workflows:
NumPy (Numerical Python)
NumPy is the powerful Python library for numerical calculations. It provides N-dimensional arrays that are much faster and more efficient than regular Python lists for handling numbers.
| Feature | Description | Relevance for Chem Eng |
|---|---|---|
| ndarray | Efficient multi-dimensional arrays | Storing experimental data, discretized model variables |
| Math Functions | Fast vectorized math operations | Bulk calculations on datasets, equation solving |
| Linear Algebra | Matrix operations, solving linear systems | Mass balances, reactor modeling |
| Random Numbers | Generation of random numbers for simulations | Monte Carlo simulations, uncertainty analysis |
In Chemical Engineering, NumPy is used to solve linear and non-linear equations for process simulations. It is also applied to solve problems involving finite difference methods, finite element analysis, and numerical integration in reactor design and heat transfer studies.
Here is an example of using the NumPy library for concentration unit conversion. Given concentration data in g/L and the molecular weight of the substance, the output is converted to mol/L.
import numpy as np
# Concentration in g/L and Molecular Weight in g/mol
concentration_g_per_L = np.array([10, 25, 50, 100]) # in g/L
molecular_weight = 18.015 # Example: Water (H2O) in g/mol
# Conversion to mol/L: (g/L) / (g/mol) = mol/L
concentration_mol_per_L = concentration_g_per_L / molecular_weight
# Display the results
print("Concentration in g/L:", concentration_g_per_L)
print("Converted Concentration in mol/L:", concentration_mol_per_L)
Output:
Concentration in g/L: [10 25 50 100]
Converted Concentration in mol/L: [0.555 1.388 2.776 5.551]Related: Heat Conduction in Spherical Shell – Online Calculator & Python Code
Related: Joule-Thomson Effect – Coefficient Calculation for CO2 and N2
SciPy (Scientific Python)
SciPy is built on top of NumPy and provides a collection of algorithms and functions for scientific and technical computing, including numerical integration, curve fitting, solving ordinary differential equations (ODEs), and more.
| SciPy Module | Functionality | Relevance for Chem Eng |
|---|---|---|
| scipy.integrate | Numerical integration, ODE solvers | Solving reactor design equations (PFR, CSTR), transient heat/mass transfer |
| scipy.optimize | Optimization algorithms, curve fitting | Finding optimal process conditions, fitting kinetic models to data |
| scipy.stats | Statistical functions, probability distributions | Analyzing experimental data, quality control, uncertainty propagation |
| scipy.interpolate | Interpolation tools | Estimating values between discrete data points (e.g., property data) |
| scipy.linalg | Advanced linear algebra routines | Solving complex systems of linear equations from models |
In chemical engineering, SciPy is used for various applications like solving reaction kinetics with ODE solvers, finding temperature distribution in heat exchangers, and designing distillation columns. SciPy makes complex engineering calculations faster and more accurate.
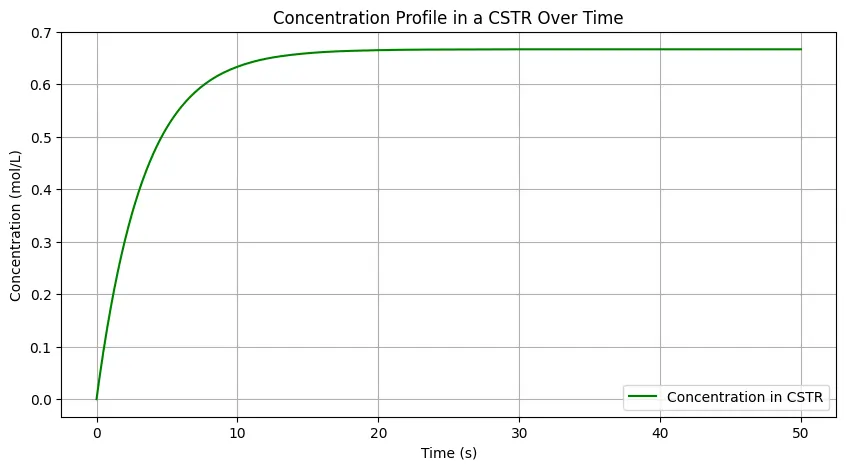
Here is an example of using SciPy library for CSTR Concentration Profile Simulation by solving ODE for the material balance of CSTR.
import numpy as np
from scipy.integrate import solve_ivp
import matplotlib.pyplot as plt
# Parameters
F = 1.0 # Flow rate (L/s)
V = 10.0 # Reactor volume (L)
k = 0.2 # Reaction rate constant (1/s)
CA0 = 2.0 # Inlet concentration (mol/L)
# ODE definition for CSTR
def cstr_ode(t, CA):
dCA_dt = (F/V) * (CA0 - CA) - k * CA
return dCA_dt
# Initial concentration and time span
CA_initial = 0.0 # Starting with no reactant in the reactor
t_span = (0, 50) # Time range in seconds
t_eval = np.linspace(0, 50, 200) # Evaluation points
# Solve the ODE
solution = solve_ivp(cstr_ode, t_span, [CA_initial], t_eval=t_eval)
# Plot the solution
plt.figure(figsize=(8, 5))
plt.plot(solution.t, solution.y[0], label="Concentration in CSTR", color='green')
plt.title("Concentration Profile in a CSTR Over Time")
plt.xlabel("Time (s)")
plt.ylabel("Concentration (mol/L)")
plt.grid(True)
plt.legend()
plt.show()Output:
Related: Why Chemical Engineers Are Switching to These Open-Source Tools
Related: 15 Mostly used Fundamental Constants Every Chemical Engineer Should Know
These Python Libraries Every Chemical Engineer Should Know for Faster Workflows
byu/ChemEnggCalc inChemicalEngineering
Matplotlib & Seaborn
Matplotlib is a plotting library that gives you complete control over every part of a figure. Seaborn is built on top of Matplotlib and provides a simpler way to create attractive and informative statistical graphs.
| Library | Used For | Chem Eng Plots |
|---|---|---|
| Matplotlib | Highly customizable, wide range of plot types | P-V-T diagrams, concentration profiles, reaction rate curves, process trends |
| Seaborn | Statistical visualizations, aesthetic defaults | Distribution plots, heatmaps, regression plots for data analysis |
In chemical engineering, Matplotlib is used to create plots like temperature vs. time, concentration profiles, and phase diagrams to understand process behavior.
Seaborn makes it easier to create more attractive and informative visualizations, like heat maps for reactor temperatures or distribution plots for experimental data.
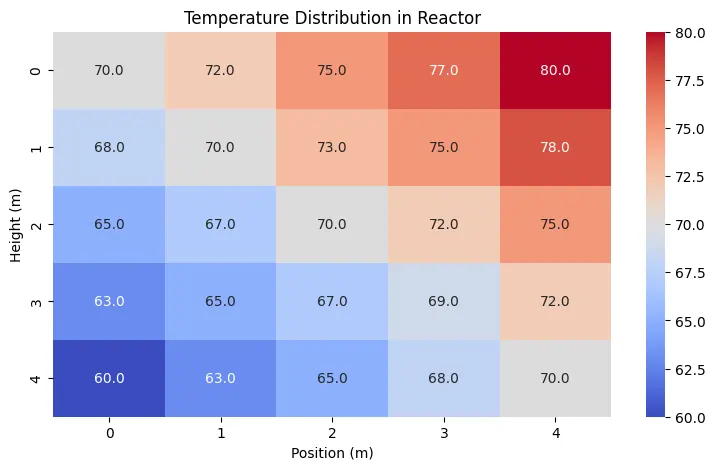
Here is an example for using seaborn library to plot a heatmap of temperature distribution in a reactor.
!pip install seaborn matplotlib
import seaborn as sns
import matplotlib.pyplot as plt
import numpy as np
# Sample data: Temperature distribution in a reactor (°C)
temperature_data = np.array([
[70, 72, 75, 77, 80],
[68, 70, 73, 75, 78],
[65, 67, 70, 72, 75],
[63, 65, 67, 69, 72],
[60, 63, 65, 68, 70]
])
# Plotting the heatmap
plt.figure(figsize=(9, 5))
sns.heatmap(temperature_data, annot=True, cmap='coolwarm', fmt=".1f")
plt.title("Temperature Distribution in Reactor")
plt.xlabel("Position (m)")
plt.ylabel("Height (m)")
plt.show()
Output:
Cantera for Thermodynamics, Kinetics & Transport
Cantera is a powerful open-source library used for solving problems involving thermodynamics, chemical kinetics, and transport processes. It’s particularly strong in reacting flows.
| Feature | Description | Chem Eng Application Examples |
|---|---|---|
| Thermodynamics | Phase equilibrium, species properties (H, S, G, Cp) | Calculating heat of reaction, dew/bubble points, flash calcs |
| Chemical Kinetics | Reaction rate evaluation, reactor network analysis | Simulating CSTRs, PFRs, flame speeds, ignition delay times |
| Transport Properties | Viscosity, thermal conductivity, diffusion coeffs | Modeling transport phenomena in reactors and separation units |
| Multi-phase Systems | Gas, liquid, surface, and interface chemistry | Heterogeneous catalysis, multiphase reactors, electrochemistry |
Here is an example for simulating the Haber Process to calculate the equilibrium temperature, pressure, and mole fractions of nitrogen, hydrogen, and ammonia using Cantera.
!pip install cantera
import cantera as ct
# Define the gas mixture (Ammonia synthesis reaction)
gas = ct.Solution('gri30.yaml')
gas.TP = 700 + 273.15, 20 * ct.one_atm # Set temperature to 700°C and pressure to 20 atm
# Set the initial mole fractions (1:3 ratio of N2 and H2)
gas.X = {'N2': 1, 'H2': 3, 'NH3': 0}
# Equilibrate the gas mixture for constant T and P
gas.equilibrate('TP')
# Display the results
print(f"Equilibrium Temperature: {gas.T:.2f} K")
print(f"Equilibrium Pressure: {gas.P / ct.one_atm:.2f} atm")
print("Equilibrium Mole Fractions:")
# Loop through species names and mole fractions
for species, fraction in zip(gas.species_names, gas.X):
if fraction > 1e-5: # Display only significant species
print(f"{species}: {fraction:.5f}")Output:
Equilibrium Temperature: 973.15 K
Equilibrium Pressure: 4.73 atm
Equilibrium Mole Fractions:
H2: 0.74919
NH3: 0.00108
N2: 0.24973Related: Relation Between Van der Waals Constants and Critical Constants
Related: Clausius Clapeyron Equation Calculator, Derivation and Applications
CoolProp for Thermophysical Properties
CoolProp provides accurate thermophysical property data for a wide range of pure fluids, pseudo-pure fluids, and predefined mixtures.
| Property Type | Examples | Chem Eng Application Examples |
|---|---|---|
| State Properties | Density, Pressure, Temperature, Enthalpy, Entropy | Cycle analysis (Rankine, Refrigeration), energy balances |
| Transport Properties | Viscosity, Thermal Conductivity, Prandtl Number | Heat exchanger design, fluid flow calculations, pressure drop |
| Saturation Properties | Vapor pressure, Saturation temperature/pressure | Condenser/evaporator design, flash calculations |
| Phase Identification | Identifying fluid phase at given T, P | Process safety, equipment design |
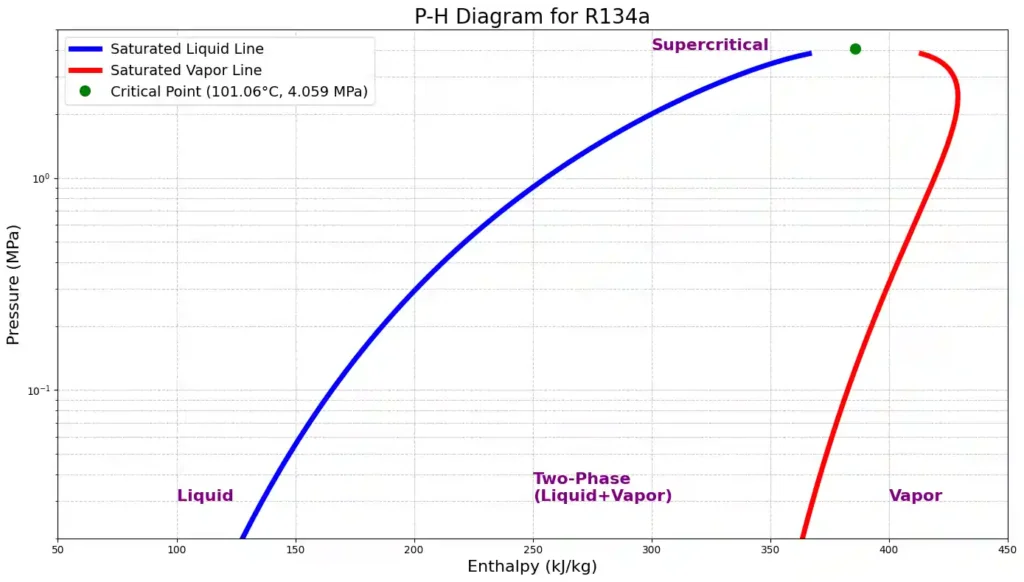
Google Colab Python code for plotting above P-H diagram for refrigerant using coolprop data – python code link
This example shows how to use the CoolProp library in python to calculate water properties (density, viscosity, specific heat, and thermal conductivity) at a specified temperature and pressure.
from CoolProp.CoolProp import PropsSI
# Properties of water at 300 K and 1 atm (101325 Pa)
T = 300 # Kelvin
P = 101325 # Pascals
density = PropsSI('D', 'T', T, 'P', P, 'Water') # Density in kg/m^3
viscosity = PropsSI('V', 'T', T, 'P', P, 'Water') # Viscosity in Pa*s
cp = PropsSI('C', 'T', T, 'P', P, 'Water') # Specific heat capacity (Cp) in J/kg*K
conductivity = PropsSI('L', 'T', T, 'P', P, 'Water') # Thermal conductivity in W/m*K
print(f"Water properties at {T} K and {P/1000} kPa:")
print(f" Density: {density:.2f} kg/m^3")
print(f" Viscosity: {viscosity:.2e} Pa*s")
print(f" Specific Heat (Cp): {cp:.2f} J/kg*K")
print(f" Thermal Conductivity: {conductivity:.3f} W/m*K")Output:
Water properties at 300 K and 101.325 kPa:
Density: 996.56 kg/m^3
Viscosity: 8.51e-04 Pa*s
Specific Heat (Cp): 4180.59 J/kg*K
Thermal Conductivity: 0.609 W/m*KRelated: Area of Cross-Section Calculator for Hollow Sections, Beams & Shapes
Related: Friction Factor Calculator Moody’s Diagram for Smooth and Rough Pipes
Related: Head Loss or Pressure Loss Calculator using Darcy-Weisbach Equation
Pint – Library for Units & Conversions
Pint is the python package to define and operate physical quantities with units. It allows you to perform calculations while keeping track of units.
| Feature | Description | Chem Eng Benefit |
|---|---|---|
| Unit Registry | Defines and manages a wide range of units | Consistent unit usage across calculations |
| Quantity Objects | Numbers associated with units | Calculations are dimensionally aware |
| Unit Conversion | Automatic and explicit unit conversions | Easily switch between SI, Imperial, and custom units |
| Dimensional Analysis | Checks for dimensional consistency | Prevents errors like adding meters to kilograms |
In Chemical Engineerig, Pint library is used to simplify unit conversions and calculations for measurements like temperature or volume. It saves time, reduces errors, and ensures consistent units in designs and teamwork.
This example shows how the Pint library manages scientific units and conversions by calculating the volume of a gas using the ideal gas law.
# Install Pint if not already installed
!pip install pint
# Import required libraries
from pint import UnitRegistry
import numpy as np
# Initialize Pint unit registry
ureg = UnitRegistry()
# Define constants and inputs
R = 8.314 * ureg.J / (ureg.mol * ureg.K) # Ideal gas constant
n = 10 * ureg.kmol # Amount of nitrogen
T = ureg.Quantity(25, ureg.degC) # Temperature
P = 5 * ureg.bar # Pressure
# Convert temperature to Kelvin and pressure to Pascals for ideal gas law
T = T.to(ureg.K) # Convert °C to K
P = P.to(ureg.Pa) # Convert bar to Pa
# Calculate volume using ideal gas law: V = nRT / P
V = (n * R * T) / P
# Convert volume to liters and cubic meters for practical use
V_liters = V.to(ureg.L) # Convert to liters
V_cubic_meters = V.to(ureg.m**3) # Convert to cubic meters (fixed syntax)
# Print results
print(f"Ideal Gas Volume Calculation for Nitrogen:")
print(f"Amount: {n:.2f}")
print(f"Temperature: {T:.2f} ({T.to(ureg.degC):.2f})")
print(f"Pressure: {P:.2e} ({P.to(ureg.bar):.2f})")
print(f"Volume: {V_cubic_meters:.2f} or {V_liters:.2f}")
# Practical application: Check if volume fits a standard tank (e.g., 10 m^3)
tank_volume = 10 * ureg.m**3 # Fixed syntax for tank volume
if V_cubic_meters <= tank_volume:
print(f"The volume {V_cubic_meters:.2f} fits in a {tank_volume:.2f} tank.")
else:
print(f"The volume {V_cubic_meters:.2f} is too large for a {tank_volume:.2f} tank.")Output:
Ideal Gas Volume Calculation for Nitrogen:
Amount: 10.00 kilomole
Temperature: 298.15 kelvin (25.00 degree_Celsius)
Pressure: 5.00e+05 pascal (5.00 bar)
Volume: 49.58 meter ** 3 or 49576.38 liter
The volume 49.58 meter ** 3 is too large for a 10.00 meter ** 3 tank.Must Read: Personal Carbon Footprint Calculator – Track your CO2 Emissions
Also Read: The Crucial Role of Chemical Engineering in Everyday Life
Also Read: Online Psychrometric Calculator for Chemical Engineers
ChemPy for Chemical Reaction Engineering
ChemPy is a Python package designed to solve chemical kinetics problems, especially systems of ODEs from reaction mechanisms. It can also be used to analyze chemical equilibria.
| Feature | Description | Chem Eng Application Examples |
|---|---|---|
| Reaction Parsing | Defines reactions from strings | Easily input complex reaction mechanisms |
| ODE System Generation | Automatically creates ODEs from reactions | Model batch reactors, CSTRs (with additional formulation) |
| Equilibrium Calculation | Solves for equilibrium compositions | Determine extent of reaction, product distribution at equil. |
| Symbolic Manipulation | Can work with symbolic species and rates | Aid in deriving rate laws or understanding mechanisms |
This example demonstrates how to use the ChemPy Python package to balance reaction stoichiometry and it can also be used to calculate the amount of reactant needed for a given chemical reaction.
# Install ChemPy if not already installed
!pip install chempy
# Import required libraries
from chempy import balance_stoichiometry
from chempy.chemistry import Substance
# Define the substances involved in the propane combustion reaction
substances = {
'C3H8': Substance.from_formula('C3H8'), # Propane
'O2': Substance.from_formula('O2'), # Oxygen
'CO2': Substance.from_formula('CO2'), # Carbon dioxide
'H2O': Substance.from_formula('H2O') # Water
}
# Define reactants and products for the reaction: C3H8 + O2 -> CO2 + H2O
reactants = ['C3H8', 'O2']
products = ['CO2', 'H2O']
# Balance the reaction stoichiometry
reac, prod = balance_stoichiometry(reactants, products, substances=substances)
# Print the balanced reaction
print("Balanced Combustion Reaction for Propane:")
print(f"{reac['C3H8']} C3H8 + {reac['O2']} O2 -> {prod['CO2']} CO2 + {prod['H2O']} H2O")Output:
Balanced Combustion Reaction for Propane:
1 C3H8 + 5 O2 -> 3 CO2 + 4 H2ORelated: Bernoulli’s Equation Calculator / Solver – Interactive Python Code
Also Read: Chemical Engineering as a career option in India
Note: Many process simulators, such as DWSIM, COCO, and Aspen Plus, can integrate with Python via dedicated interfaces or scripts, enabling users to automate tasks and easily customize simulations.
The integration of Python into chemical engineering is changing the field by making problem-solving faster, more flexible, data-focused, and innovative. Learning these libraries gives you a strong and adaptable set of tools.
Resources
- Process Control and Automation. Chemical Engineering Fundamentals Review
- Seider, W. D., Seader, J. D., & Lewin, D. R. (2010). Product and Process Design Principles: Synthesis, Analysis, and Evaluation. John Wiley & Sons.
- Aspen Technology. (n.d.). Aspen Plus and HYSYS for Process Modeling. AspenTech.
- The MathWorks, Inc. (n.d.). MATLAB and Simulink for Chemical Process Control. MathWorks.
- Roush, J. (2020). Data Science and Machine Learning in Chemical Engineering. AIChE.
Disclaimer: The content provided here is for educational purposes. While efforts ensure accuracy, results may not always reflect real-world scenarios. Verify results with other sources and consult professionals for critical applications. Contact us for any suggestions or corrections.