Table of Contents
Understanding the behavior of real gases are important in the field of chemical engineering, Real gases deviate from ideal gas behavior due to the finite size of molecules and the presence of intermolecular forces.
The Van der Waals Equation of State and other real gas models are useful to take care of these factors and provides more accurate description of gas behavior under non-ideal conditions.
What is Van der Waals Equation of State?
The Van der Waals Equation is first practical cubic equation of state which describes the behavior of real gases. Unlike Ideal Gas Law, this equation accounts for the finite size of molecules and the attraction between them. It provides more accurate prediction about the behavior of real gases, especially under high pressure and low temperature conditions.
The Van der Waals Equation of State is given by:
\[\left(P + \frac{an^2}{V^2}\right) (V – nb) = nRT\]
where:
- P is the pressure of the gas,
- Vm is the molar volume of the gas,
- T is the temperature,
- n is the no. of moles of gas,
- R is the universal gas constant,
- a is a measure of the attraction between particles,
- b is the volume occupied by the gas molecules.
Key Points:
- The purpose of the term \(\frac{a}{V_m^2}\) is to account for the attractive forces between molecules, which make the pressure lower than that which would be exerted in the ideal-gas state.
- The purpose of constant \(b\) is to account for the finite size of molecules, which makes the volume larger than in the ideal-gas state.
Related: Relation Between Van der Waals Constants and Critical Constants
Related: Clausius Clapeyron Equation Calculator, Derivation and Applications
Van der Waals Equation of State Calculator
This web tool is used to calculate the thermodynamic properties like Pressure, Volume, Temperature, or Number of Moles of a chemical compound based on the Van der Waals equation. The value of calculated volume is based on assumption where simplified the equation by putting the PV=nRT and converted the cubic equation into simpler form (calculations are given below).
Note: Data table for constant values (a and b) are provided in the table below, identify the chemical from the list and put the correct value of a and b.
Related: Kirchoff’s Law of Thermal Radiation, Wien’s Displacement Law
Related: Joule-Thomson Effect – Coefficient Calculation for CO2 and N2
Van der Waals Constant (a and b values) – Data Table
Here are the following table lists the Van der Waals constants for various compounds including a number of common gases and volatile liquids.
Data Source: Van der Waals constant – a and b values
| Compound Name | a (Pa·m⁶/mol²) | b (m³/mol) |
|---|---|---|
| Xenon | 0.425 | 0.00005105 |
| Water | 0.5536 | 0.00003049 |
| Trimethylamine | 1.337 | 0.0001101 |
| Trifluoromethane | 0.5378 | 0.000064 |
| Trichloromethane | 1.534 | 0.0001019 |
| Toluene | 2.438 | 0.0001463 |
| Tin tetrachloride | 2.727 | 0.0001642 |
| Thiophene | 1.721 | 0.0001058 |
| Tetrahydrofuran | 1.639 | 0.0001082 |
| Tetrafluorosilane | 0.5259 | 0.0000724 |
| Tetrafluoromethane | 0.404 | 0.0000633 |
| Tetrafluoroethylene | 0.6954 | 0.0000809 |
| Tetrachlorosilane | 2.096 | 0.000147 |
| Tetrachloromethane | 2.001 | 0.0001281 |
| Sulfur hexafluoride | 0.7857 | 0.0000879 |
| Sulfur dioxide | 0.6803 | 0.00005636 |
| Silicon tetrafluoride | 0.4251 | 0.00005571 |
| Silane | 0.4377 | 0.00005786 |
| Radon | 0.6601 | 0.00006239 |
| Pyrrole | 1.882 | 0.0001049 |
| Pyridine | 1.977 | 0.0001137 |
| Propene | 0.8442 | 0.0000824 |
| Propane | 0.8779 | 0.00008445 |
| Phosphine | 0.4692 | 0.00005156 |
| Phenol | 2.293 | 0.0001177 |
| Pentane | 1.926 | 0.000146 |
| Ozone | 0.357 | 0.0000487 |
| Oxygen | 0.1382 | 0.00003186 |
| Octane | 3.788 | 0.0002374 |
| Nitrous oxide | 0.3832 | 0.00004415 |
| Nitrogen trifluoride | 0.358 | 0.0000545 |
| Nitrogen dioxide | 0.5354 | 0.00004424 |
| Nitrogen | 0.137 | 0.0000387 |
| Nitric oxide | 0.1358 | 0.00002789 |
| Neopentane | 1.717 | 0.0001411 |
| Neon | 0.02135 | 0.00001709 |
| Methylamine | 0.7106 | 0.0000588 |
| Methanol | 0.9649 | 0.00006702 |
| Methane | 0.2253 | 0.00004278 |
| Mercury | 0.82 | 0.00001696 |
| Krypton | 0.2349 | 0.00003978 |
| Isobutane | 1.332 | 0.0001164 |
| Iodobenzene | 3.352 | 0.0001656 |
| Hydrogen sulfide | 0.449 | 0.00004287 |
| Hydrogen selenide | 0.5338 | 0.00004637 |
| Hydrogen iodide | 0.6309 | 0.000053 |
| Hydrogen fluoride | 0.9565 | 0.0000739 |
| Hydrogen cyanide | 1.129 | 0.0000881 |
| Hydrogen chloride | 0.3716 | 0.00004081 |
| Hydrogen bromide | 0.451 | 0.00004431 |
| Hydrogen | 0.02476 | 0.00002661 |
| Hydrazine | 0.846 | 0.0000462 |
| Hexane | 2.471 | 0.0001735 |
| Heptane | 3.106 | 0.0002049 |
| Helium | 0.00346 | 0.0000238 |
| Germanium tetrachloride | 2.29 | 0.0001485 |
| Furan | 1.274 | 0.0000926 |
| Freon | 1.078 | 0.0000998 |
| Fluoromethane | 0.4692 | 0.00005264 |
| Fluorobenzene | 2.019 | 0.0001286 |
| Fluorine | 0.1171 | 0.000029 |
| Ethylene | 0.4612 | 0.0000582 |
| Ethylamine | 1.074 | 0.00008409 |
| Ethyl acetate | 2.072 | 0.0001412 |
| Ethanol | 1.218 | 0.00008407 |
| Ethanethiol | 1.139 | 0.00008098 |
| Ethane | 0.5562 | 0.0000638 |
| Dodecane | 6.938 | 0.0003758 |
| Dimethyl sulfide | 1.304 | 0.00009213 |
| Dimethyl ether | 0.818 | 0.00007246 |
| Diethyl sulfide | 1.9 | 0.0001214 |
| Diethyl ether | 1.761 | 0.0001344 |
| Decane | 5.274 | 0.0003043 |
| Cyclopropane | 0.834 | 0.0000747 |
| Cyclohexane | 2.311 | 0.0001424 |
| Cyanogen | 0.7769 | 0.00006901 |
| Chloromethane | 0.757 | 0.00006483 |
| Chloroethane | 1.105 | 0.00008651 |
| Chlorobenzene | 2.577 | 0.0001453 |
| Chlorine | 0.6579 | 0.00005622 |
| Carbon tetrachloride | 1.97483 | 0.0001281 |
| Carbon monoxide | 0.1505 | 0.00003985 |
| Carbon disulfide | 1.177 | 0.00007685 |
| Carbon dioxide | 0.364 | 0.00004267 |
| Butane | 1.466 | 0.0001226 |
| Bromobenzene | 2.894 | 0.0001539 |
| Benzene | 1.824 | 0.0001193 |
| Argon | 0.1355 | 0.00003201 |
| Aniline | 2.914 | 0.0001486 |
| Ammonia | 0.4225 | 0.0000371 |
| Acetylene | 0.4516 | 0.0000522 |
| Acetonitrile | 1.781 | 0.0001168 |
| Acetone | 1.602 | 0.0001124 |
| Acetic anhydride | 2.0158 | 0.0001263 |
| Acetic acid | 1.77098 | 0.0001065 |
| 2-Propanol | 1.582 | 0.0001109 |
| 2-Butanone | 1.997 | 0.0001326 |
| 1-Propanol | 1.626 | 0.0001079 |
| 1-Pentanol | 2.588 | 0.0001568 |
| 1-Octanol | 4.471 | 0.0002442 |
| 1-Hexanol | 3.179 | 0.0001856 |
| 1-Heptanol | 3.817 | 0.000215 |
| 1-Dodecanol | 7.57 | 0.000375 |
| 1-Decanol | 5.951 | 0.0003086 |
| 1-Butanol | 2.094 | 0.0001326 |
| 1-1-1-Trichloroethane | 2.015 | 0.0001317 |
Pressure and Volume Calculation for Van der Waals Equation
The Van der Waals equation is an equation applies to the Real Gas Law but limited to temperature and pressure conditions, it includes two constants, a and b, to account for deviations from ideal behavior.
The calculator we have presented above makes the calculation for Pressure, Volume, Temperature and number of moles. Here we will clarify how the van der waals equation is rearranged to get the calculated value.
For Pressure calculation, the volume, temperature and the number of moles of the gas molecule are known, the pressure can be calculated using formula:
\[P=\frac{nRT}{V-nb} – \frac{n^2a} {V^2}\]
For Volume calculation of a real gas, V in term \(\frac{n^2a} {V^2}\) can be approximated as \(\frac{nR}{TP}\) as ideal gas. Therefore, V can be calculated as:
\[V= \frac{nR^3T^3}{PR^2T^2 + aP^2}+ nb\]
If we solve the cubic equation form of van der waals equation, it will lead to 3 roots, it can be further divided as 3 real roots or 1 real & 2 imaginary roots, which defines the plot on the PV isotherm.
Related: Hydraulic Diameter Calculator for Circular and Non-Circular cross-section
Related: Inverse Square Law for Radiation
Limitations of Van der Waals’ Equation
Van der Waals’ equation becomes invalid under actual condition as discussed below :
- The values of a and b (which are assumed to be constant) are found to vary with temperature. Thus the results obtained from the equation are incorrect when the variation of a and b is large with respect to temperature.
- The equation is not accurate enough in the critical region.
Example Problem on Van der Waals Equation
Carbon dioxide gas (1.00 mole) at 373 K occupies 536 mL at 50.0 atm pressure. What is the calculated value of the pressure using:
- The Ideal Gas Equation
- The Van der Waals Equation
Data given for Van der Waals constants for carbon dioxide: a = 3.61 L2 atm mol-2 & b = 0.0428 L/mol
Calculate the percentage deviation of each value from the observed pressure.
1. Using the Ideal Gas Equation
Given:
- V = 0.536 L
- n = 1.00 mol
- T = 373 K
The Ideal Gas Law is given by \(P = \frac{nRT}{V}\)
Solving for ( P ):
\(P = \frac{1.00 \times 0.0821 \times 373}{0.536}\)
\(P = 57.1 \, \text{atm}\)
The actual pressure is:
\(\text{Actual pressure} = 50.0 \, \text{atm}\)
The percentage deviation is calculated as:
% deviation \( = \left(\frac{7.1}{50.0}\right) \times 100 = 14.2 \%\)
2. Using the Van der Waals Equation
The Van der Waals equation is given by:
\(P=\frac{nRT}{V-nb} – \frac{n^2a} {V^2}\)
on substituting and solving the values we get, P = 49.6 atm
The percentage deviation is calculated as:
\( = \left(\frac{0.4}{50.0}\right) \times 100 = 0.8 \%\)
Therefore, using the Ideal Gas Law, the calculated pressure is 57.1 atm, resulting in a percentage deviation of 14.2% from the observed pressure of 50.0 atm. Using the Van der Waals equation, the calculated pressure is 49.6 atm, which has a percentage deviation of 0.8% from the observed pressure.
Also Read: Online Psychrometric Calculator for Chemical Engineers
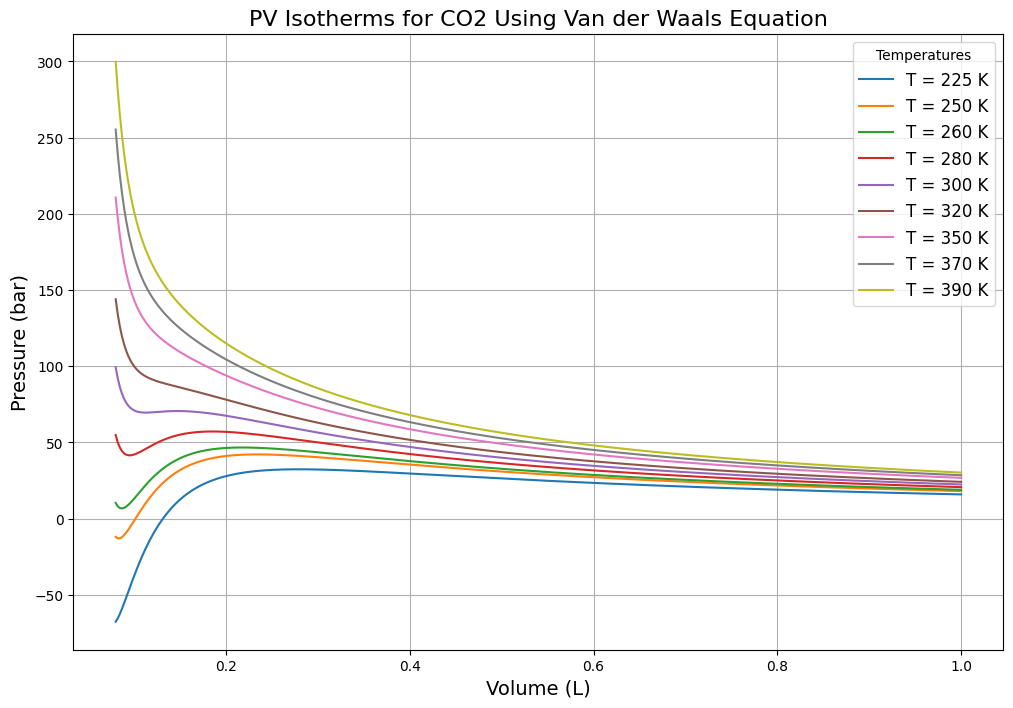
Python Code for Van der Waals- PV Isotherm
This Python code helps user to calculate and visualizes the Pressure-Volume (PV) isotherms for carbon dioxide (CO₂) gas using the Van der Waals equation. Plots are generated at different temperatures which shows the relationship between pressure and volume changes with temperature.
Note: This Python code solves the specified problem. Users can copy the code and run it in a suitable Python environment. By adjusting the input parameters, users can observe how the output changes accordingly.
import numpy as np
import matplotlib.pyplot as plt
# Van der Waals constants for CO2
R = 0.08314 # L·bar·K⁻¹·mol⁻¹, gas constant
a = 3.640 # L²·bar·mol⁻²
b = 0.04267 # L·mol⁻¹
# Define the temperatures (in Kelvin) for the isotherms
temperatures = [225, 250, 260, 280, 300, 320, 350, 370, 390] # temperatures
# Create an array of volume values
V = np.linspace(0.08, 1, 400) # Volume in liters
# Create the plot
plt.figure(figsize=(12, 8))
for T in temperatures:
# Van der Waals equation: P = [RT / (V - b)] - [a / (V^2)]
P = (R * T) / (V - b) - (a / (V ** 2))
# Plotting the isotherm
plt.plot(V, P, label=f'T = {T} K')
# Plots
plt.xlabel('Volume (L)', fontsize=14)
plt.ylabel('Pressure (bar)', fontsize=14)
plt.title('PV Isotherms for CO2 Using Van der Waals Equation', fontsize=16)
plt.legend(title='Temperatures', fontsize=12)
plt.grid(True)
plt.show()
Output:
Resources
- Heat Transfer a Practical Approach – Book by Yunus A Çengel
- “Thermodynamics: An Engineering Approach” by Yunus A. Çengel and Michael A. Boles
- “Introduction to Chemical Engineering Thermodynamics” by J.M. Smith, H.C. Van Ness, and M.M. Abbott
- NPTEL Lectures on Thermodynamics
- Engineering Thermodynamics: A Textbook – by P.K Nag
Disclaimer: The Solver provided here is for educational purposes. While efforts ensure accuracy, results may not always reflect real-world scenarios. Verify results with other sources and consult professionals for critical applications. Contact us for any suggestions or corrections.


Everything said made a lott of sense. However, what about this?
suppose you addwd a little information? I am not saying your
information is not good, however suppose you added a post title to possibly grb a person’s attention? I mean Van der
Waals Equation Calulator and PV Isotherm for Reall
Gases – ChemEnggCalc is a little vanilla.
You might peek at Yahoo’s home page aand see howw they crrate post headlines to grab people interested.
You might try adding a video or a related pic or two to grab readeers interested about everything’ve written.
In my opinion, it could make your posts a little biit more interesting. https://lvivforum.pp.ua