Table of Contents
What is Prandtl number?
The Prandtl number (\( \text{Pr} \)) is a dimensionless number named after the German physicist Ludwig Prandtl. It is defined as the ratio of momentum diffusivity to thermal diffusivity. This number gives the relationship between kinematic viscosity and thermal diffusivity of the fluid.
Mathematically, the Prandtl number is given by:
\[\text{Pr} = \frac {\text{Momentum Diffusivity}} {\text{Thermal Diffusivity}}\]
\[\text{Pr} = \frac{\nu}{\alpha} = \frac{\mu \, C_p}{k}\]
where:
- \(\nu\) is the momentum diffusivity (kinematic viscosity) (m²/s)
- \(\alpha\) is the thermal diffusivity (m²/s)
- \(\mu\) is the dynamic viscosity (N·s/m²)
- \(C_p\) is the specific heat (J/kg·K)
- \(k\) is the thermal conductivity (W/m·K)
Note: The mass transfer analog of the Prandtl number (Pr) is the Schmidt Number.
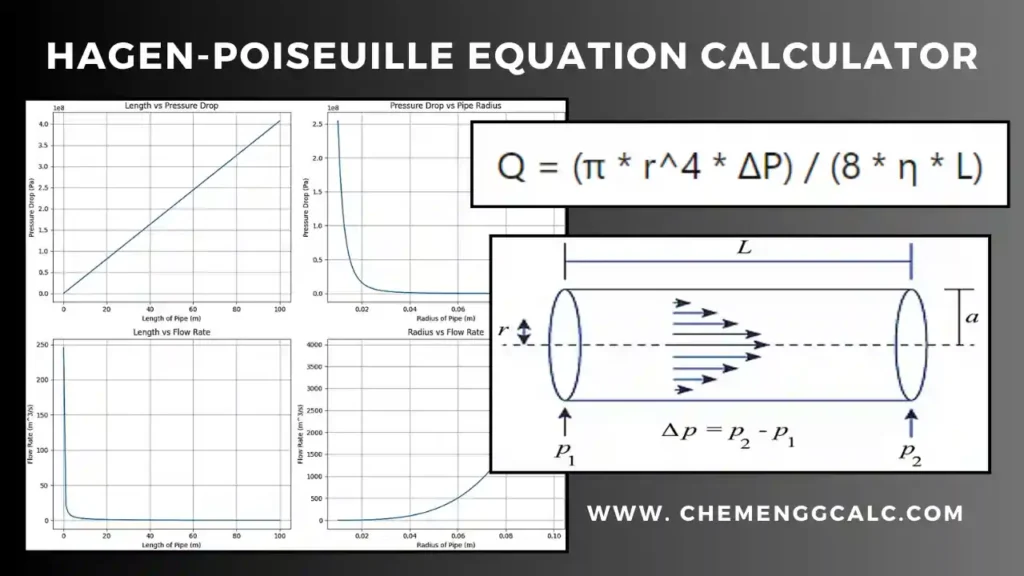
Related: Hydraulic Diameter Calculator for Circular and Non-Circular cross-section
Related: Inverse Square Law for Radiation
Prandtl Number Calculator
This calculator is helpful to calculate parameters like \(Pr\) Number, dynamic viscosity (μ), thermal conductivity (k), or specific heat capacity \(C_P\) based on the formula \(\text{Pr} = \frac{\mu \, C_p}{k}\) This tool is useful for engineers and scientists working in fields involving heat transfer and fluid dynamics.
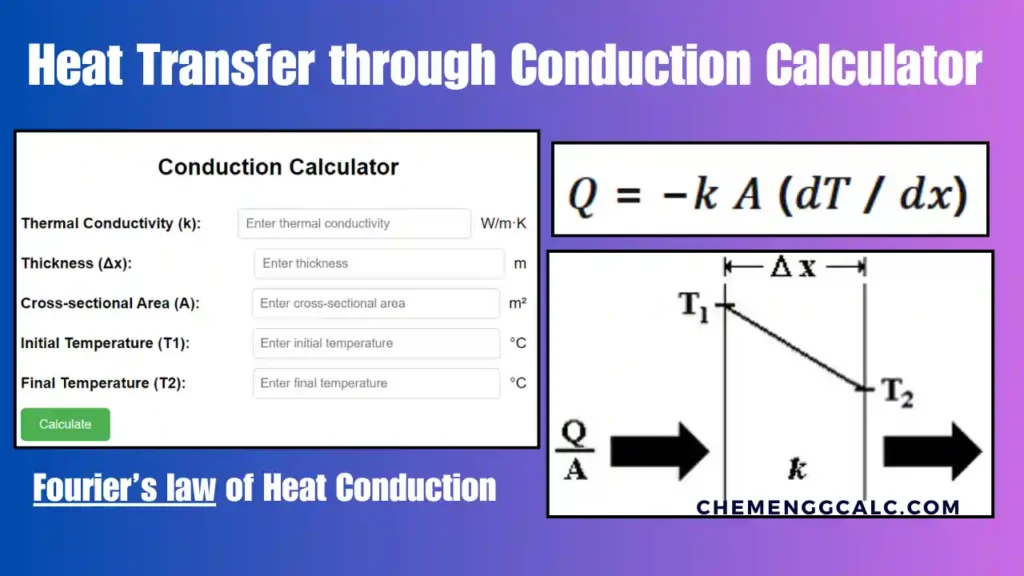
Related: Heat Transfer through Conduction Calculator – Fourier’s law
Prandtl Number Significance
The Prandtl number \(Pr\) is significant in Heat Transfer and Fluid Mechanics. It provides a measure of the relative rates of momentum and heat transport by diffusion in a fluid.
– Fluid Behavior Characterization
- The \(Pr\) number indicates the relative thickness of the velocity boundary layer to the thermal boundary layer.
- Low Prandtl number (Pr≪1): Thermal diffusivity dominates, meaning heat diffuses much faster than momentum (for liquid metals).
- High Prandtl number (Pr≫1): Momentum diffusivity dominates, meaning momentum diffuses much faster than heat (for oils)
– Boundary Layer Characteristics
- In fluids, Low \(Pr\) means, the thermal boundary layer is much thicker than the velocity boundary layer.
- In fluids, High \(Pr\) means the velocity boundary layer is thicker than the thermal boundary layer.
– Heat Transfer Analysis
- In convective heat transfer, \(Pr\) number helps to predict the pattern and efficiency of heat transfer.
- Along with Reynolds Number, it also calculates the Nusselt Number which characterizes the convective heat transfer coefficient.
Typical Ranges for Prandtl Number for various Fluids
Air at room temperature has a \(Pr = 0.71 \) and for water at 18°C it is around 7.56, which means that the thermal diffusivity is more dominant for air than for water.
For many fluids, \(\text{Pr}\) lies in the range from 1 to 10. For gases, \(\text{Pr}\) is generally about 0.7. For liquid metals the Prandtl number is very small, generally in the range from 0.01 to 0.001.
Source: Wikipedia – Prandtl Number – Experimental Values at specific temperature-Pressure conditions provided in the table below:
| Substance | Temperature/Condition | Prandtl_Number (Pr) |
|---|---|---|
| Molten potassium | 975 K | 0.003 |
| Mercury | Room temperature | ~0.015 |
| Molten lithium | 975 K | 0.065 |
| Noble gas mixtures | Various | 0.16–0.7 |
| Oxygen | Room temperature | 0.63 |
| Air and many other gases | Room temperature | ~0.71 |
| Gaseous ammonia | Room temperature | 1.38 |
| R-12 refrigerant | Various | 4–5 |
| Water | 18 °C | 7.56 |
| Seawater | 0 °C | 13.4 |
| Seawater | 20 °C | 7.2 |
| n-Butanol | Room temperature | 50 |
| Engine oil | Various | 100–40,000 |
| Glycerol | Room temperature | 1000 |
| Polymer melts | Various | 10,000 |
| Earth’s mantle | Various | ~1×10²⁵ |
Other Dimensionless Numbers
Here we have tabulated the list of dimensionless numbers which are commonly used in Chemical Engineering with their formula, definition, and significance.
| Dimensionless Number | Formula | Definition | Significance |
|---|---|---|---|
| Reynolds Number (Re) | \[\text{Re} = \frac{\rho u L}{\mu} = \frac{u L}{\nu}\] | Ratio of inertial forces to viscous forces. | Determines flow regime: Re < 2000: Laminar flow Re > 4000: Turbulent flow. Critical for pipe flow and reactors. |
| Prandtl Number (Pr) | \[\text{Pr} = \frac{C_p \mu}{k}\] | Ratio of momentum diffusivity to thermal diffusivity. | It indicates the relative thickness of momentum and thermal boundary layers in heat transfer. |
| Nusselt Number (Nu) | \[\text{Nu} = \frac{h L}{k}\] | Ratio of convective to conductive heat transfer. | It is used in convective heat transfer problems to evaluate heat transfer coefficients. |
| Sherwood Number (Sh) | \[\text{Sh} = \frac{k_m L}{D}\] | Ratio of convective to diffusive mass transfer. | It is important in mass transfer, especially in diffusion and absorption processes. |
| Péclet Number (Pe) | \[\text{Pe} = \text{Re} \cdot \text{Pr}\] | Ratio of advection to diffusion. Combines Reynolds and Prandtl numbers. | It is important in heat and mass transfer processes where advection dominates over diffusion. |
| Schmidt Number (Sc) | \[\text{Sc} = \frac{\mu}{\rho D}\] | Ratio of momentum diffusivity to mass diffusivity. | It is used in mass transfer to relate momentum and mass transport. Analogous to the Prandtl number in heat transfer. |
| Lewis Number (Le) | \[\text{Le} = \frac{\alpha}{D} = \frac{\text{Sc}}{\text{Pr}}\] | Ratio of thermal diffusivity to mass diffusivity. | It relates thermal and mass transport; Le = 1 implies similar thermal and mass diffusivity. |
| Fourier Number (Fo) | \[\text{Fo} = \frac{\alpha t}{L^2}\] | Ratio of heat conduction rate to heat storage rate. | It is used in unsteady-state heat transfer problems, like transient conduction. |
| Biot Number (Bi) | \[\text{Bi} = \frac{h L}{k}\] | Ratio of internal thermal resistance to surface thermal resistance. | It determines whether heat conduction is lumped or distributed. Bi < 0.1: Lumped system approximation valid. |
| Weber Number (We) | \[\text{We} = \frac{\rho u^2 L}{\sigma}\] | Ratio of inertial forces to surface tension forces. | It is important in multiphase flows, droplet formation, and fluid interface stability. |
| Capillary Number (Ca) | \[\text{Ca} = \frac{\mu u}{\sigma}\] | Ratio of viscous forces to surface tension forces. | It is used to study the behavior of capillary flows and thin-film phenomena. |
| Froude Number (Fr) | \[\text{Fr} = \frac{u^2}{g L}\] | Ratio of inertial forces to gravitational forces. | It is important in free-surface flows, mixing, and fluidized bed design. |
| Damköhler Number (Da) | \[\text{Da} = \frac{\text{Reaction rate}}{\text{Flow rate}}\] | Ratio of chemical reaction time to residence time. | It is important in chemical reaction engineering; determines if a process is reaction- or transport-limited. |
| Thiele Modulus (ϕ) | \[\phi = L \sqrt{\frac{k}{D}}\] | Ratio of reaction rate to diffusion rate within a catalyst particle. | It is used in heterogeneous catalysis to determine diffusion limitations within catalyst pores. |
| Knudsen Number (Kn) | \[\text{Kn} = \frac{\lambda}{L}\] | Ratio of molecular mean free path to characteristic length. | It is important in gas flow through small pores or microchannels; indicates continuum vs. molecular flow regime. |
| Eckert Number (Ec) | \[\text{Ec} = \frac{u^2}{C_p \Delta T}\] | Ratio of kinetic energy to enthalpy difference. | It is used in high-speed flows and heat transfer problems involving viscous dissipation. |
| Grashof Number (Gr) | \[\text{Gr} = \frac{g \beta \Delta T L^3}{\nu^2}\] | Ratio of buoyancy forces to viscous forces. | It determines the importance of natural convection in heat transfer problems. |
| Stanton Number (St) | \[\text{St} = \frac{h}{\rho u C_p}\] | Ratio of heat transfer coefficient to the product of fluid velocity and specific heat. | It is used in heat transfer to describe convective heat transfer efficiency relative to bulk flow. |
Related: 10 Mostly used Dimensionless Numbers in Chemical Engineering
Example Problem on Prandtl Number
Calculate the Prandtl number of ethyl alcohol at 300K. Refer to Perry’s for the following values: dynamic viscosity (μ) in Pa·s, thermal conductivity (k) in W/(m·K), and specific heat capacity (Cp) in J/(kg·K)?
To solve the given problem, we need to calculate the \( \text{Pr} \) for ethyl alcohol at 300K using the provided properties:
Given:
- Dynamic viscosity (\( \mu \)) = 1.2 Pa·s
- Thermal conductivity (\( k \)) = 0.16 W/(m·K)
- Specific heat capacity (\( C_p \)) = 2440 J/(kg·K)
Using formula, \( \text{Pr} = \frac{\mu \cdot C_p}{k} \)
Substituting values into the formula,
\( \text{Pr} = \frac{1.2 \, \text{Pa·s} \times 2440 \, \text{J/(kg·K)}}{0.16 \, \text{W/(m·K)}} \)
\( \text{Pr} = \frac{1.2 \times 2440}{0.16} \)
\( \text{Pr} = \frac{2928}{0.16} \)
\( \text{Pr} = 18,300 \)
Therefore, the \(Pr\) number of ethyl alcohol at 300K is 18,300.
Resources
- “Perry’s Chemical Engineers’ Handbook” by Don W. Green and Marylee Z. Southard
- “Fundamentals of Heat and Mass Transfer” by Frank P. Incropera, David P. DeWitt, Theodore L. Bergman, and Adrienne S. Lavine
- “Fluid Mechanics” by Frank M. White
Disclaimer: The Solver provided here is for educational purposes. While efforts ensure accuracy, results may not always reflect real-world scenarios. Verify results with other sources and consult professionals for critical applications. Contact us for any suggestions or corrections.