Table of Contents
What is Biot Number?
Biot Number (Bi) is a dimensionless quantity used in heat transfer calculations. It is defined as internal thermal resistance within a body (conduction) to the external thermal resistance due to convection.
The Biot number provides a measure of the temperature drop in the solid relative to the temperature difference between the surface and the fluid.
Mathematically, it is defined as,
\[Bi = \frac{\text{Internal Thermal Resistance}}{\text{External convective Resistance}}\]
\[Bi = \frac{R_{\text{Conduction}}}{R_{\text{convection}}} = \frac{hL_c}{k}\]
where:
- \( h \) is the convective heat transfer coefficient (W/m2·K)
- \(L_c \) is the characteristic length (m)
- \( k \) is the thermal conductivity of the material (W/m·K)
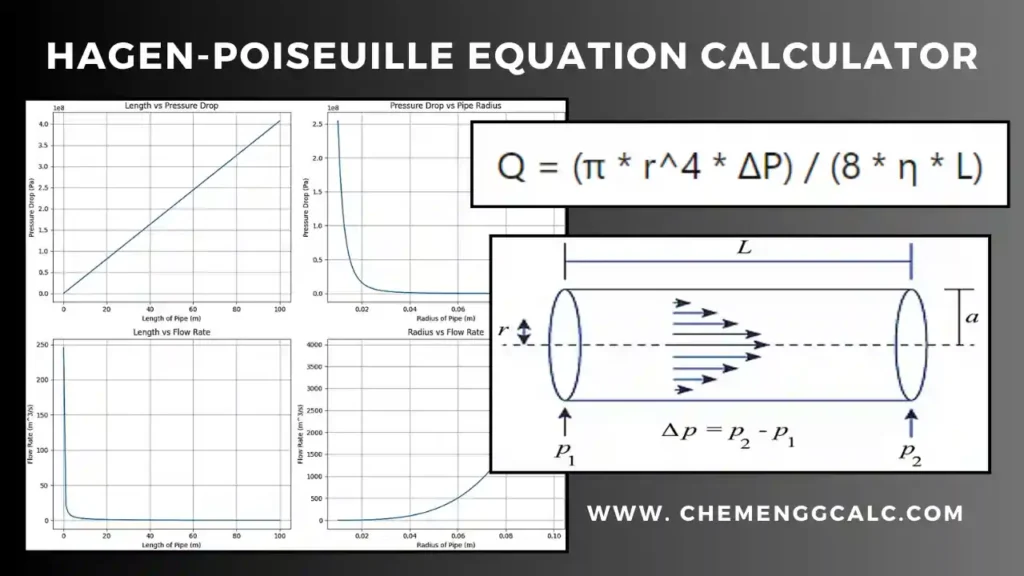
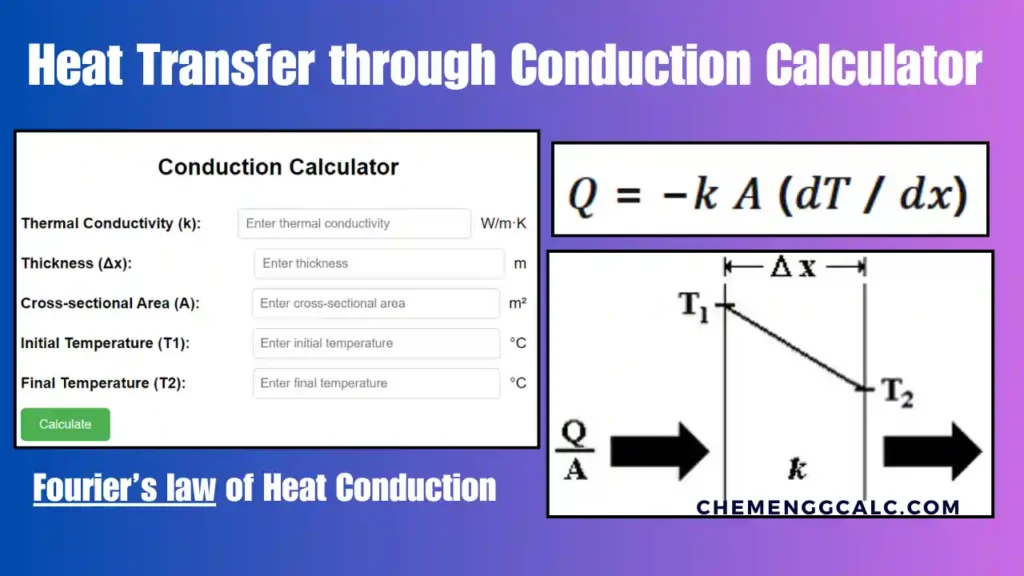
Related: Heat Transfer through Conduction Calculator – Fourier’s law
Biot Number Calculator
This Calculator / web tool helps user to the Biot number. User inputs the values for the convective heat transfer coefficient \(h\), the characteristic length \(L_c \), and the thermal conductivity \(k\).
Related: Hydraulic Diameter Calculator for Circular and Non-Circular cross-section
Related: Inverse Square Law for Radiation
Physical Significance of Biot Number
Bi Number is significant in Heat Transfer Calculations, it compares internal thermal resistance within a solid to the external thermal resistance due to convection.
Consider a steady-state temperature distribution in plane wall with surface convection as shown in figure below.
Low Biot Number (\( Bi \ll 1 \))
The resistance to conduction within the solid is much less than the resistance to convection. The temperature within the solid is relatively uniform because heat conduction inside the solid is very effective compared to the convective heat transfer at the surface.
High Biot Number (\( Bi \gg 1 \))
The resistance due to conduction is much larger than the resistance due to convection. The temperature difference across the solid is much larger than that between the surface and the fluid.
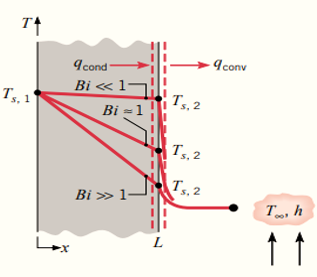
The Bi number is important in determining the mechanism of heat transfer and helps in simplifying the analysis of thermal systems.
Example: Consider a small hot metal sphere, when immersed in a pool of water, conduction within a sphere and convection at the sphere surface will be influenced by the water.
If the Bi number is low, the temperature sphere is assumed to be uniform. If the Bi number is high it means that temperature gradient exists.
Other Dimensionless Numbers
Here we have tabulated the list of dimensionless numbers which are commonly used in Chemical Engineering with their formula, definition, and significance.
| Dimensionless Number | Formula | Definition | Significance |
|---|---|---|---|
| Reynolds Number (Re) | \[\text{Re} = \frac{\rho u L}{\mu} = \frac{u L}{\nu}\] | Ratio of inertial forces to viscous forces. | Determines flow regime: Re < 2000: Laminar flow Re > 4000: Turbulent flow. Critical for pipe flow and reactors. |
| Prandtl Number (Pr) | \[\text{Pr} = \frac{C_p \mu}{k}\] | Ratio of momentum diffusivity to thermal diffusivity. | It indicates the relative thickness of momentum and thermal boundary layers in heat transfer. |
| Nusselt Number (Nu) | \[\text{Nu} = \frac{h L}{k}\] | Ratio of convective to conductive heat transfer. | It is used in convective heat transfer problems to evaluate heat transfer coefficients. |
| Sherwood Number (Sh) | \[\text{Sh} = \frac{k_m L}{D}\] | Ratio of convective to diffusive mass transfer. | It is important in mass transfer, especially in diffusion and absorption processes. |
| Péclet Number (Pe) | \[\text{Pe} = \text{Re} \cdot \text{Pr}\] | Ratio of advection to diffusion. Combines Reynolds and Prandtl numbers. | It is important in heat and mass transfer processes where advection dominates over diffusion. |
| Schmidt Number (Sc) | \[\text{Sc} = \frac{\mu}{\rho D}\] | Ratio of momentum diffusivity to mass diffusivity. | It is used in mass transfer to relate momentum and mass transport. Analogous to the Prandtl number in heat transfer. |
| Lewis Number (Le) | \[\text{Le} = \frac{\alpha}{D} = \frac{\text{Sc}}{\text{Pr}}\] | Ratio of thermal diffusivity to mass diffusivity. | It relates thermal and mass transport; Le = 1 implies similar thermal and mass diffusivity. |
| Fourier Number (Fo) | \[\text{Fo} = \frac{\alpha t}{L^2}\] | Ratio of heat conduction rate to heat storage rate. | It is used in unsteady-state heat transfer problems, like transient conduction. |
| Biot Number (Bi) | \[\text{Bi} = \frac{h L}{k}\] | Ratio of internal thermal resistance to surface thermal resistance. | It determines whether heat conduction is lumped or distributed. Bi < 0.1: Lumped system approximation valid. |
| Weber Number (We) | \[\text{We} = \frac{\rho u^2 L}{\sigma}\] | Ratio of inertial forces to surface tension forces. | It is important in multiphase flows, droplet formation, and fluid interface stability. |
| Capillary Number (Ca) | \[\text{Ca} = \frac{\mu u}{\sigma}\] | Ratio of viscous forces to surface tension forces. | It is used to study the behavior of capillary flows and thin-film phenomena. |
| Froude Number (Fr) | \[\text{Fr} = \frac{u^2}{g L}\] | Ratio of inertial forces to gravitational forces. | It is important in free-surface flows, mixing, and fluidized bed design. |
| Damköhler Number (Da) | \[\text{Da} = \frac{\text{Reaction rate}}{\text{Flow rate}}\] | Ratio of chemical reaction time to residence time. | It is important in chemical reaction engineering; determines if a process is reaction- or transport-limited. |
| Thiele Modulus (ϕ) | \[\phi = L \sqrt{\frac{k}{D}}\] | Ratio of reaction rate to diffusion rate within a catalyst particle. | It is used in heterogeneous catalysis to determine diffusion limitations within catalyst pores. |
| Knudsen Number (Kn) | \[\text{Kn} = \frac{\lambda}{L}\] | Ratio of molecular mean free path to characteristic length. | It is important in gas flow through small pores or microchannels; indicates continuum vs. molecular flow regime. |
| Eckert Number (Ec) | \[\text{Ec} = \frac{u^2}{C_p \Delta T}\] | Ratio of kinetic energy to enthalpy difference. | It is used in high-speed flows and heat transfer problems involving viscous dissipation. |
| Grashof Number (Gr) | \[\text{Gr} = \frac{g \beta \Delta T L^3}{\nu^2}\] | Ratio of buoyancy forces to viscous forces. | It determines the importance of natural convection in heat transfer problems. |
| Stanton Number (St) | \[\text{St} = \frac{h}{\rho u C_p}\] | Ratio of heat transfer coefficient to the product of fluid velocity and specific heat. | It is used in heat transfer to describe convective heat transfer efficiency relative to bulk flow. |
Related: 10 Mostly used Dimensionless Numbers in Chemical Engineering
Python Code for Biot Number calculation
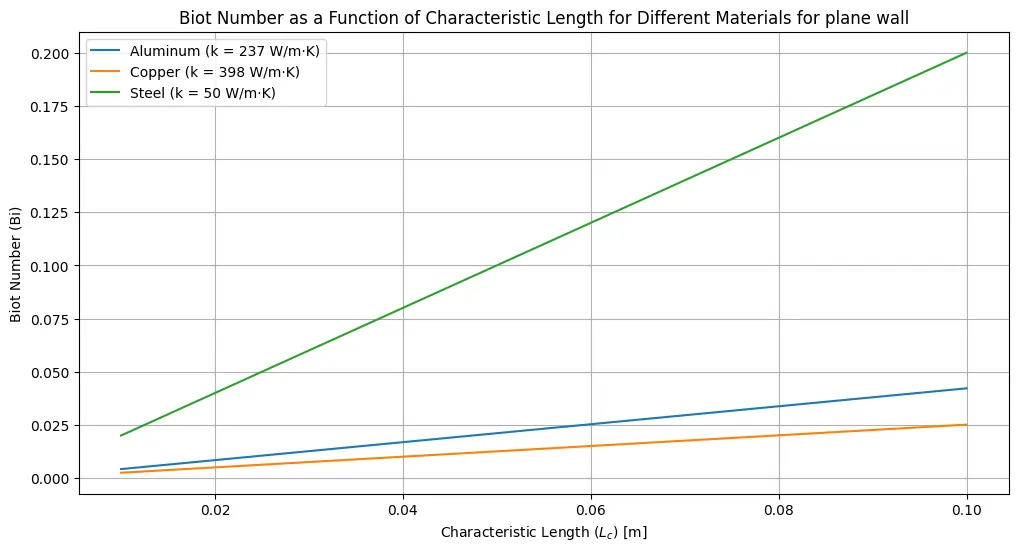
This Python code helps user to find the Bi number for different materials and convective heat transfer coefficient of 100 W/m²·K. It calculates the values of Bi at different Characteristic length for Aluminum, Copper and Steel.
Note: This Python code solves the specified problem. Users can copy the code and run it in a suitable Python environment. By adjusting the input parameters, and observe how the output changes accordingly.
import numpy as np
import matplotlib.pyplot as plt
def calculate_biot_number(h, L_c, k):
"""
Calculate the Biot_number for a plane wall.
Parameters:
h (float): Convective heat transfer coefficient (W/m²·K)
L_c (float): Characteristic length (m)
k (float): Thermal conductivity of the material (W/m·K)
Returns:
float: The Biot number
"""
return (h * L_c) / k
# Example data
h = 100 # W/m²·K, convective heat transfer coefficient
# Thermal conductivities for different materials
thermal_conductivities = {
'Aluminum': 237, # W/m·K
'Copper': 398, # W/m·K
'Steel': 50 # W/m·K
}
# Range of characteristic lengths
L_c_values = np.linspace(0.01, 0.1, 100) # Characteristic lengths from 0.01m to 0.1m
# Plotting
plt.figure(figsize=(12, 6))
for material, k in thermal_conductivities.items():
biot_numbers = calculate_biot_number(h, L_c_values, k)
plt.plot(L_c_values, biot_numbers, label=f'{material} (k = {k} W/m·K)')
plt.xlabel('Characteristic Length ($L_c$) [m]')
plt.ylabel('Biot Number (Bi)')
plt.title('Biot Number as a Function of Characteristic Length for Different Materials for plane wall')
plt.grid(True)
plt.legend()
plt.show()
# Display Biot numbers for specific characteristic lengths
for material, k in thermal_conductivities.items():
for example_L_c in [0.02, 0.05, 0.08]:
biot_number_example = calculate_biot_number(h, example_L_c, k)
print(f"Biot Number for {material} with L_c = {example_L_c} m: {biot_number_example:.4f}")Output:
Resources
- “Fundamentals of Heat and Mass Transfer” by Theodore L. Bergman, Adrienne S. Lavine, Frank P. Incropera, and David P. DeWitt.
- “Heat Transfer Book” by David W. Hahn.
- “Conduction of Heat in Solids” by H.S. Carslaw and J.C. Jaeger.
- “Introduction to Heat Transfer” by Frank P. Incropera, David P. DeWitt, Theodore L. Bergman, and Adrienne S. Lavine.
Disclaimer: The Solver provided here is for educational purposes. While efforts ensure accuracy, results may not always reflect real-world scenarios. Verify results with other sources and consult professionals for critical applications. Contact us for any suggestions or corrections.