Table of Contents
What is Antoine Equation?
The Antoine equation is a mathematical expression (empirical correlation) used to describe the non-linear thermodynamic relationship between the vapour pressure of a pure substance and its temperature. This equation is derived from Clausius Clapeyron Equation.
To find the vapour pressure of liquids and some solids over a range of temperatures. The equation is given by:
\[\log_{10}(P) = A – \frac{B}{C + T}\]
where:
- \(P\) is the vapor pressure.
- \(T\) is the temperature.
- \(A\), \(B\), and \(C\) are substance-specific constants.
The constants \(A\), \(B\), and \(C\) are determined empirically and are different for each substance.
Also Read: Online Psychrometric Calculator for Chemical Engineers
Related: Joule-Thomson Effect – Coefficient Calculation for CO2 and N2
Antoine Equation Calculator for Vapour Pressure
This vapor pressure calculator uses the Antoine equation to calculate the vapor pressure of various chemicals (given in dropdown menu) with predefined Antoine coefficients (A, B, C) and temperature (in degrees Celsius), the calculator displays the vapor pressure in mmHg.
Related: Hydraulic Diameter Calculator for Circular and Non-Circular cross-section
Related: Inverse Square Law for Radiation
Antoine Equation Coefficients for pure compounds
Here we have provided the mostly used Coefficient of Antoine Equation for pure compounds. Data is arranged in the alphabetical order. The Antoine Coefficient should only be used within the given temperature range Tmin and Tmax.
Data source: Hydrocarbon Processing, 68(10), p65-68, 1989. (Yaws, C. L. and Yang, H. C.)
| Formula | Compound Name | A | B | C | TMIN (deg C) | TMAX (deg C) |
|---|---|---|---|---|---|---|
| C3H6O | acetone | 7.2316 | 1277.03 | 237.23 | -32 | 77 |
| C2H4O2 | acetic-acid | 7.2996 | 1479.02 | 216.82 | 17 | 157 |
| C2H3N | acetonitrile | 7.0735 | 1279.2 | 224.01 | -13 | 117 |
| C6H6 | benzene | 6.90565 | 1211.033 | 220.79 | -16 | 104 |
| C4H10 | butane | 6.80896 | 935.86 | 238.73 | -78 | 19 |
| CCL4 | carbon-tetrachloride | 6.8941 | 1219.58 | 227.17 | -20 | 101 |
| C6H5Cl | chlorobenzene | 6.9781 | 1431.05 | 217.56 | 47 | 147 |
| C4H9Cl | 1-chlorobutane | 6.9379 | 1227.43 | 224.11 | -18 | 112 |
| C4H9Cl | 2-chlorobutane | 6.9447 | 1195.8 | 226.01 | -23 | 102 |
| CHCL3 | chloroform | 6.9371 | 1171.2 | 227 | -13 | 97 |
| C6H12 | cyclohexane | 6.8413 | 1201.531 | 222.647 | 6 | 105 |
| C5H10 | cyclopentane | 6.88676 | 1124.162 | 231.361 | -40 | 72 |
| C10H22 | decane | 6.96375 | 1508.75 | 195.374 | 58 | 203 |
| CH2Cl2 | dichloromethane | 7.0803 | 1138.91 | 231.46 | -44 | 59 |
| C4H10O | ethyl-ether | 6.92032 | 1064.07 | 228.8 | -61 | 20 |
| C4H8O2 | p-dioxane | 7.0063 | 1288.5 | 211.01 | 2 | 137 |
| C20H42 | eicosane | 7.1522 | 2032.7 | 132.1 | 198 | 379 |
| C2H6O | Ethyl-alcohol (ethanol) | 8.2133 | 1652.05 | 231.48 | -3 | 96 |
| C8H10 | ethylbenzene | 6.95719 | 1424.255 | 213.206 | 26 | 163 |
| C2H6O2 | ethylene-glycol | 8.7945 | 2615.4 | 244.91 | 91 | 221 |
| C7H16 | heptane | 6.89385 | 1264.37 | 216.636 | -2 | 123 |
| C6H14 | hexane | 6.87024 | 1168.72 | 224.21 | -25 | 92 |
| CH4O | methanol | 8.0724 | 1574.99 | 238.87 | -16 | 91 |
| C4H8O2 | ethyl-acetate | 7.0146 | 1211.9 | 216.01 | -13 | 112 |
| CH3NO2 | nitromethane | 7.044 | 1291 | 209.01 | 5 | 136 |
| C9H20 | nonane | 6.9344 | 1429.46 | 201.82 | 39 | 179 |
| C8H18 | octane | 6.9094 | 1349.82 | 209.385 | 19 | 152 |
| C5H12 | pentane | 6.87632 | 1075.78 | 233.205 | -50 | 58 |
| C6H6O | phenol | 7.1345 | 1516.07 | 174.57 | 72 | 208 |
| H20 | Water (liquid) | 8.07131 | 1730.63 | 233.426 | 1 | 100 |
| H20 | Water (vapour) | 8.14019 | 1810.94 | 244.485 | 99 | 374 |
Example Problem on Antoine Equation
Given the Antoine equation for benzene, \[\log_{10} P_{\text{sat}} = 6.90 – \frac{1211}{220.8 + T}\] where \(P_{\text{sat}}\) is the saturation pressure in torr and (T) is the temperature in °C, estimate the heat of vaporization of benzene at \(60^\circ\text{C}\).
Given equation is the generalised form of Antoine Equation which can be interpreted to calculate the values of \(P_{sat}\), we get the following table on calculation (assuming heat of vaporization \( \Delta H_{\text{vap}} \) is constant in this narrow region of 60 °C).
| Temp \((^\circ\text{C})\) | \(P_{sat}\) | \(ln_{P_{sat}}\) | \(\frac{1}{T}\) (1/K) |
|---|---|---|---|
| 55 | 322.9513139 | 5.777501581 | 0.00304878 |
| 65 | 460.0141266 | 6.131257199 | 0.00295858 |
using the Clausius-Clapeyron equation,
Related: Read out our full article on Clausius-Clapeyron equation
\[\ln P_{\text{sat}} = -\frac{\Delta H_{\text{vap}}}{R} \left(\frac{1}{T}\right) + C\]
here,
- \( P_{\text{sat}} \) is the saturation pressure,
- \( \Delta H_{\text{vap}} \) is the enthalpy of vaporization,
- \( R \) is the universal gas constant,
- \( T \) is the temperature in Kelvin,
- \( C \) is a constant of integration.
The plot for the values of \(\ln P_{\text{sat}}\) vs \(\frac{1}{T}\) is plotted below and slope of this line is calculated, based on the given slope value, \( \Delta H_{\text{vap}} \) is calculated.
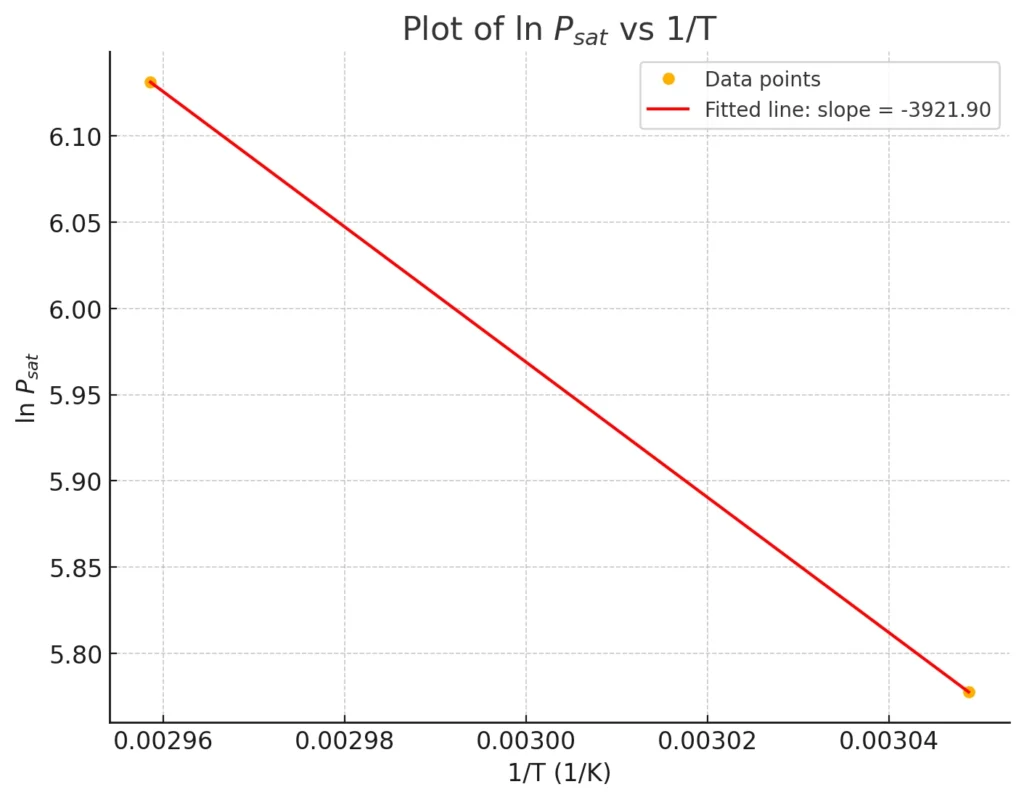
we get, the slope value from plot is -3921.90
therefore, the value of \(-\frac{\Delta H_{\text{vap}}}{R} = -3921.90\)
on calculation, \(\Delta H_{\text{vap}} = 32606.4\) J/mol which is equal to 32.6 KJ/mol.
The heat of vaporization of benzene at \(60^\circ\text{C}\) is 32.6 KJ/mol.
Related: Van der Waals Equation Calculator and PV Isotherm for Real Gases
Must Read: Personal Carbon Footprint Calculator – Track your CO2 Emissions
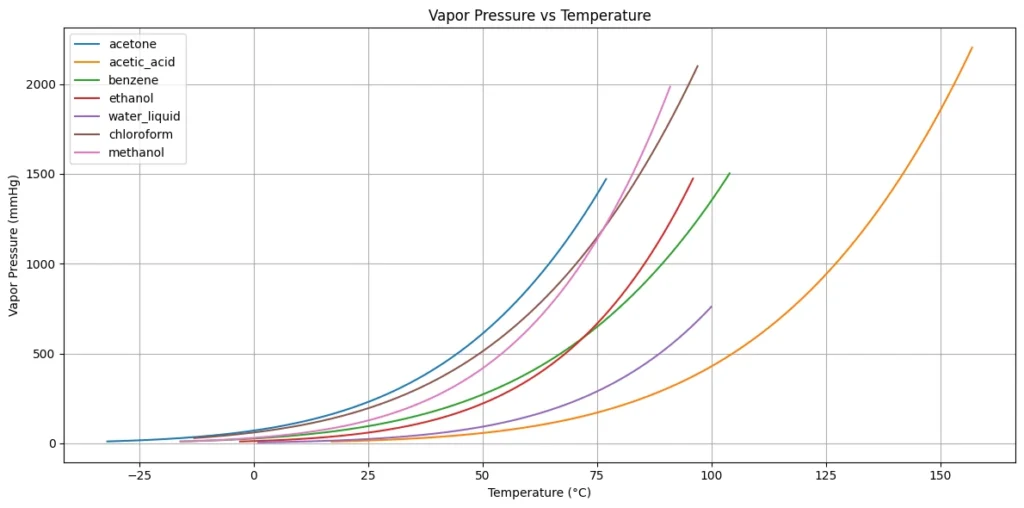
Python Code for Vapor Pressure Calculation
This python code helps user to calculate the Vapor Pressure using Antoine Equation versus temperature based on coefficients A, B, and C specific to each chemical, which are provided in the chemicals_data dictionary for the given temperature range.
Note: This Python code solves the specified problem. Users can copy the code and run it in a suitable Python environment. By adjusting the input parameters, users can observe how the output changes accordingly.
import numpy as np
import matplotlib.pyplot as plt
# Function to calculate vapor pressure using Antoine equation
def antoine_equation(A, B, C, T):
return 10**(A - (B / (C + T)))
# Data for chemicals (A, B, C from Antoine equation)
chemicals_data = {
'acetone': {'A': 7.2316, 'B': 1277.03, 'C': 237.23, 'temp_range': (-32, 77)},
'acetic_acid': {'A': 7.2996, 'B': 1479.02, 'C': 216.82, 'temp_range': (17, 157)},
'benzene': {'A': 6.90565, 'B': 1211.033, 'C': 220.79, 'temp_range': (-16, 104)},
'ethanol': {'A': 8.2133, 'B': 1652.05, 'C': 231.48, 'temp_range': (-3, 96)},
'water_liquid': {'A': 8.07131, 'B': 1730.63, 'C': 233.426, 'temp_range': (1, 100)},
'chloroform': {'A': 6.9371, 'B': 1171.2, 'C': 227, 'temp_range': (-13, 97)},
'methanol': {'A': 8.0724, 'B': 1574.99, 'C': 238.87, 'temp_range': (-16, 91)}
}
# Plotting vapor pressure versus temperature for each chemical
plt.figure(figsize=(12, 6))
for chemical, data in chemicals_data.items():
A = data['A']
B = data['B']
C = data['C']
temp_min, temp_max = data['temp_range']
# Create temperature range within the specified range for each chemical
temperature_range = np.linspace(temp_min, temp_max, 500)
# Calculate vapor pressure
vapor_pressure = antoine_equation(A, B, C, temperature_range)
# Plot vapor pressure versus temperature
plt.plot(temperature_range, vapor_pressure, label=chemical)
plt.title('Vapor Pressure vs Temperature')
plt.xlabel('Temperature (°C)')
plt.ylabel('Vapor Pressure (mmHg)')
plt.legend()
plt.grid(True)
plt.tight_layout()
plt.show()Output:
Antoine Equation Excel Calculator
You can use this Excel data sheet for quick calculations. Enter the temperature in the designated orange cell and select the chemical of your choice. To simplify the process, we have listed approximately 700 chemicals.
When a chemical is selected, the corresponding A, B, and C coefficients will be automatically retrieved, and the applicable temperature range for that chemical will be displayed. The vapor pressure at the given temperature will then be calculated using the Antoine Equation.
Resources
- “Thermodynamics: An Engineering Approach” by Yunus A. Çengel and Michael A. Boles
- LIBRARY OF PHYSICO-CHEMICAL PROPERTY DATA – Data Source Link
- “Introduction to Chemical Engineering Thermodynamics” by J.M. Smith, H.C. Van Ness, and M.M. Abbott
- NPTEL Lectures on Thermodynamics
- Antoine Coefficient Data source
- Antoine equation coefficients published in the NIST Chemistry WebBook for a variety of common chemicals
Disclaimer: The Solver provided here is for educational purposes. While efforts ensure accuracy, results may not always reflect real-world scenarios. Verify results with other sources and consult professionals for critical applications. Contact us for any suggestions or corrections.



Hello! I just wanted to drop by and say that I absolutely loved this blog post. The insights you provided were incredibly helpful and thought-provoking. It’s always a pleasure to read content that is both informative and engaging. Keep up the great work, and I look forward to your next post!
Hello to every one, it’s truly a good for me to pay a quick visit this web page, it consists of useful Information.